All solid and tangible bodies consist of matter collected in atoms and molecules, but between the particles there is a space in which there seems to be nothing. If we follow this logic, then it will turn out that the atoms themselves consist of emptiness.
Inside atoms are electrons—point particles that do not have dimensions, and the nucleus of an atom is about 10 thousand times smaller than the atom itself. It turns out that any atom almost entirely consists of emptiness—most of the substance of an atom is concentrated in its nucleus. The nucleus, in turn, consists of neutrons and protons, and they are composed of even more elementary parts, which are called quarks. And quarks don’t have dimensions either—they’re just dots.

Such reasoning is based on wrong assumptions. In fact, emptiness does not exist—at the level of the microcosm (one trillionth of a millimeter), there are at least three phenomena that make thinking about emptiness meaningless.
The substance has no clear boundaries and generally consists of waves.
The behavior of molecules, atoms, and individual particles like neutrons and protons is described by quantum mechanics, with its own laws. One of the basic rules of quantum mechanics is the Heisenberg uncertainty principle. In a free interpretation, it sounds like this:
It is impossible to know the location of a particle and its speed with the same accuracy. The more the speed of a particle is determined, the more blurred its location is, and vice versa.
This limitation is fundamental; it does not depend on the quality of the measuring instruments. To determine the speed of a particle as accurately as possible, you need to observe it for some time. Under such conditions, one can only say about the location of the particle, “Well, it can be found somewhere in this area.”
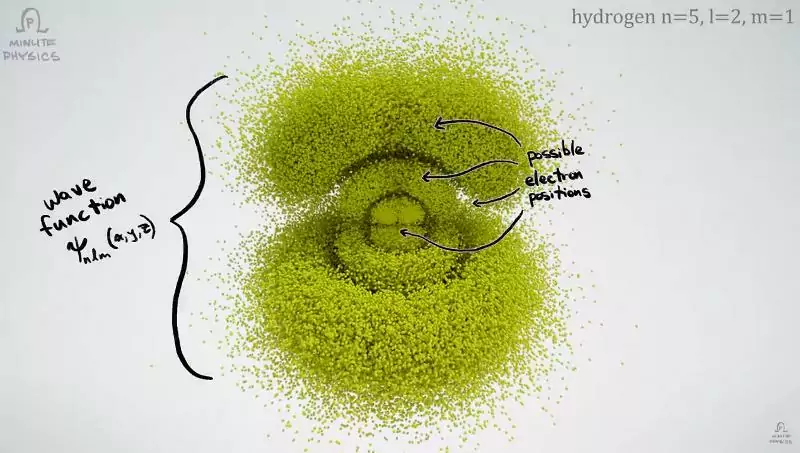
The uncertainty principle is a consequence of the dual nature of any particle of matter. The same electron is both a particle and a wave at the same time. Like any wave, it is “blurred” in space. Therefore, in the atom it is depicted not as a point but as a whole cloud. Where the brightness of the cloud is lower, the electron is less common, but the probability of its being there is never equal to zero.
The “vagueness” of microparticles is not an assumption dictated by the imperfection of scientific equipment but a fundamental property of matter. An electron is a point particle, but only when it is fixed (measured). Until the electron is fixed, it “spreads out” like a wave. The electron does not fly as a point somewhere in this wave-it is a wave with indefinite boundaries and sizes.
When atoms are assembled into molecules, their electron clouds can cross and overlap each other. So it is impossible to say with certainty that there is absolutely empty space between atoms. There can always be an electron. The uncertainty principle can “throw” it into even the most “inconvenient” areas; it’s just that the probability of this is extremely small.

There are “photographs” and even whole “video recordings” of atoms, where they look like clearly defined balls. But these are not real images of atoms as they are, but just visualizations built on the collected data. On such visualizations, the location and boundaries of atoms are always depicted “on average”. Otherwise, a clear picture would turn into a blurry mess.
The larger the material body, the more definite its boundaries and location are. Therefore, a single particle can “fill up” an entire room (and even the Universe; this is not forbidden), and bodies that consist of a huge number of particles (chairs, cars, and houses) do not blur and occupy a completely finite and definite place.
However, even large bodies are not spared from quantum “vagueness”. Their wave component is simply reduced so much that it becomes invisible. But it can be detected with high-precision equipment. So, at the LIGO observatory, using lasers, scientists recorded the quantum “trembling” of a 40-kilogram mirror—it fluctuated within one millionth of one billionth of a millimeter.
Virtual particles-“glue” of the universe
Let an electron with a non-zero probability appear anywhere—after all, there are “dark” regions in the atom where the probability of finding an electron is extremely small. That is, in these areas most of the time, there are no electrons, as well as protons and neutrons. So it’s completely empty?
And again, no. Even in the most “dark” area between the nucleus of an atom and its electron, there is something quite material-a “tether”, with the help of which the nucleus holds the electron in the atom. This “tether” is a stream of virtual photons that scurry non-stop between the nucleus and the electron.
Virtual particles are so called because they cannot be fixed directly. They disappear too quickly, decaying or turning into other particles in about one trillionth of a second. The distance they have time to move is comparable to their “vagueness” due to the uncertainty principle. This allows virtual particles to break some of the laws of physics.
“Normal” particles like real electrons and photons obey the basic laws of physics; their momentum is uniquely related to energy, and they cannot take energy from nowhere. But virtual particles can appear on their own, contrary to the law of conservation of energy; they can have a negative or imaginary mass. All this is complete nonsense from the point of view of physics. However, there are many signs of the existence of virtual particles.
These “irregular” particles are truly omnipresent—they are constantly born and immediately disappear at all points in space: between atoms, inside atoms, and even inside microparticles. Moreover, they have time to “transmit information” from one real particle to another if they are close enough. as in the case of an electron and a proton in the nucleus of an atom.

Inside the proton itself, the role of virtual particles becomes even more noticeable. The proton consists of quarks—fundamental particles that are held together by a “glue” of virtual gluons, particles that carry the strong interaction. In the same place, right inside the proton, there is a “raging sea” of virtual quarks, which constantly appear and disappear, making some contribution to the mass of the proton.
The existence of such particles may seem strange and unnatural: how can something natural violate the laws of nature itself? The reason is the principle of uncertainty. At very small distances and time intervals, some physical proportions can “break down”, because energy, mass, and momentum, as it were, do not have time to take on certain values.
The whole world is a disturbance of quantum fields.
The duality of matter, where an electron or photon is both a particle and a wave, may seem like a very far-fetched and clumsy concept. This is the fault of quantum mechanics: for all its mathematical accuracy, it describes the essence of matter rather poorly, because it tries to “cross a hedgehog with a snake”—a classical (macroscopic) picture of the world with a microscopic one.
But you can “go down a level” and move on to quantum field theory; it completely sweeps aside classical ideas about reality. In this theory, there are no longer particles as individual points or very small balls. Everything that exists here is presented in the form of quantum fields, and any particles are only perturbations of these fields, local bursts of energy.

In this case, the idea of absolute emptiness falls away as untenable, even if one closes one’s eyes to the uncertainty of the position of real particles and to the constant “seething” of virtual particles. Any quantum field is a completely monolithic material entity that fills every point of space and has non-zero energy at every point – the energy of vacuum.
This approach allows us to take a different look at the existence of virtual particles. Due to the uncertainty principle, the fields are constantly fluctuating, creating the illusion of the birth of particles. Far from always, these are normal, full-fledged particles. As a rule, field fluctuations give rise to “defective freaks” with “broken” properties. In our universe, such particles do not live long-they are called virtual.

The closer the properties of a born particle are to the “physical ideal”, the longer it lives. Those particles that are called real are just lucky to have normal, proportional properties that comply with the laws of physics. Therefore, the difference between real and virtual particles is purely quantitative. In fact, all this is one and the same, only the former undergo “natural selection”, and the latter do not.
Matter is everywhere; emptiness does not exist.
Quantum fields are literally “sewn” into the space itself and fill it. In a sense, this is the real, fundamental matter of our universe. What people are used to seeing in everyday life is just a wave “ripple” of quantum fields. Assuming that there is nothing between the particles is like looking at mountain peaks and thinking that there is an endless void between them because a veil of clouds hides the earth below.
The concept of emptiness in physics is generally rather arbitrary, which demonstrates the Unruh effect. Its description reads: if you start accelerating fast enough, then particles of warm gas will suddenly appear from the “emptiness”. That is, the “emptiness” of the environment depends on the acceleration of the observer, which is quite unusual and completely contrary to human intuition.
And although the Unruh effect has not yet been experimentally confirmed, it shows well how helpless a person’s attempts to judge emptiness and matter can be outside of their everyday reality, which makes up a very, very small part of the Universe.